Catalog Number: HUVEC-56
Name: Human Umbilical Vein Endothelial Cells, expressing a dominant negative element of p53, (HUVEC-56)
Species: Human
Amount: 0.3-0.5 millions/vial
Form: CRYOPRESERVED
Viability: not less than 70%
Product specifications
Freshly isolated HUVEC cells on earlier passages were transduced with GSE56 bearing plasmid and selected on neomycin. Overexpression of GSE56 (p53 gene fragment corresponding to the C-terminal portion of p53 (aa 275-368)) results in inhibition of p53 transactivation (Ossovskaya et al. (1996) Proc Natl Acad Sci U S A 93, 10309-10314) and cellular immortality.
DAPCEL™ HUVEC-56 guaranteed through 40 and possibly more passages.
DAPCEL™ HUVEC-56 are resistant to G418.
DAPCEL™ HUVEC-56 were tested negative for mycoplasma, bacteria, yeast, and fungi. HUVEC-56 are also negative for HIV-1, HIV-2, HBV, and HCV (by PCR).
DAPCEL™ HUVEC-56 are negative for smooth muscle a-actin (by specific staining).
DAPCEL™ HUVEC-56 are positive for von Willebrand Factor and for uptake of the DiI-Ac-LDL.
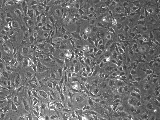
DAPCEL™ HUVEC-56, passage 17
Shipping
Product is shipped on dry ice. For maximum viability, use cells as soon as possible.
If necessary, store in liquid nitrogen vapor phase.
Product application and Storage
Applications:
Cryopreserved HUVEC are intended for use by researchers investigating the molecular mechanisms of various processes including but not limited to Angiogenesis (1,2), Cellular Adheision and organization (3), Oxidative Stress (4) and Cardiovascular-related complications associated with various diseases (5). In addition, they may serve as excellent model system to investigate Cellular Proliferation (6), Hypoxia and Inflammation related pathways (7,8); they could be also used for Drug Screening in cell based assays (9), as well as for Cell Chemotaxis and Cell Mobility/Migration assays (10).
1. Mehta et al, (2011) Angiogenesis, 355-369.
2. Takino et al (2012) World J Gastroenterol, 1781-1788.
3. Kakinoki J (2013) Biomater Sci Polym Ed., 1320-1332.
4. Sheikh-Ali et al. (2010) Diabetes Res Clin Pract, 161-166.
5. Simón-Yarza et al. (2013) Int J Pharm., 784-790.
6. Bentley et al. (2012) J. Biol. Chem., 22142-2250.
7. Zhu et al. (2008) Life Sci., 801-809.
8. Ashki (2014) Invest Ophthalmol Vis Sci., 1637-1646.
9. Wong and Fiscus (2015) Anticancer Res., 273-281.
10.Dao et al., (2013) J Transl Med., 81.
Preparation for usage:
Warning!
Handling human derived products is potentially biohazardous. Although each cell strain tests negative for HIV, HBV and HCV DNA, diagnostic tests are not necessarily 100% accurate, therefore, proper precautions must be taken to avoid inadvertent exposure. Always wear gloves and safety glasses when working with these materials. Never mouth pipette. We recommend following the universal procedures for handling products of human origin as the minimum precaution against contamination (1). 1. Grizzle and Polt (1988) “Guidelines to avoid personal contamination by infective agents in research laboratories that use human tissues.” J Tissue Culture Methods, 11, 191-199.
Materials needed to make the complete growth medium:
ATCC Vascular Cell Basal Medium, Cat # PCS-100-030
Glutamax (Life Technologies, Cat # 35050061),
Nonessential Amino Acids (Life Technologies (Cat # 11140050),
Sodium Piruvate (Life Technologies, Cat # 11360070),
Heat inactivated FBS (Life Technologies, Cat # 16000-044),
ECGS (BD Biosciences, Cat # 356006; FisherScientific, Cat # CB-40006B),
Heparin (Sigma, Cat # H4784-1G),
Pen-Strep (Life Technologies, Cat # 15140-122).
To make the complete growth medium:
Add the following components to the base ATCC Vascular Cell Basal Medium: 2 mM glutamax, 1% (v/v) nonessential amino acids, 1% (v/v) sodium pyruvate, 0.1 mg/mL heparin, 0.03-0.05 mg/ml endothelial cell growth supplement (ECGS); 20% heat inactivated fetal bovine serum. Add 50 U/mL of penicillin and 50 mg/mL of streptomycin if needed. Keep the complete medium at +4 °C. Warm the complete medium to Room Temperature (RT) when ready to work with cells.
Thawing:
1. Prepare a 37 °C water bath.
2. Keep all samples frozen until the bath is ready.
3. Place the vials into the water bath, being careful not to submerge below the lid.
4. When only a small amount of ice remains, remove the vials and dry with a lab tissue. Clean the top of the vial with a lab tissue moistened with 70% alcohol; avoid wiping away the labeling.
6. Within about 30 sec., slowly add one 1 mL of complete medium (containing serum) to the thawed cells.
7. Slowly add thawed cells to 8 mL of medium containing serum. Invert tube 2 or 3 times to mix or mix gently by pipetting up and down once.
8. Centrifuge for 8-10 min at 1000 rpm.
9. Aspirate or decant the supernatant and gently resuspend the cell pellet in 10 mL of medium.
10. Remove an aliquot for cell count and proceed with experimental manipulations.
11. Culture the cells in complete medium. Every 2-3 days change media to fresh one.
Passaging and freezing:
Split HUVEC when cells reach sub confluent state (see photo above; ~90% of confluency).
Quickly remove media, wash cells with HANK’s solution (Sigma, Cat # H6648); for splitting use Enzyme free cell dissociation solution, HANK’s based (Millipore, Cat # S-004-C), incubate at 37 °C; after cells will be detached from plate, (examine the flask under a microscope for a successful cell harvest), collect them in to a tube and spin down for 8-10 min at 1000 rpm.
Discard supernatant and resuspend cells in complete media. Grow HUVEC in cell culture incubator (37 °C / 5% CO2). Avoid use of PBS and Trypsin for splitting HUVECs. Do not split at more than 1:2 ratio.
HUVEC-56 must be frozen in a complete cell culture media, supplied with 7% DMSO (AMRESCO, ultra pure grade, Cat # WN182) in a sufficient concentration (5x106/mL).
To grow HUVEC-56 in Serum Free Experimental conditions we would highly recommend the use of PeproGrow Endothelial Cell Basal Medium (PeproGrow-MacroV) from PeproTech, Cat # ENDO-BM.
Storage:
3 weeks or less: -80 °C
Long term: Liquid Nitrogen
Stability:
Cannot bee guaranteed if stored improperly.